How Can the Concentration Be Described Using Different Units
Such a small concentration is also expressed as 58 g per 10 6 g 58 ppm of. It is important to note that molarity is per liters of solution not per liters of solvent.

Units Of Concentration Dilute Concentrated Solution Embibe
So if you substract your y-intercept from the absorbance and divide by the slope you are finding the concentration of your sample.

. Solution concentration can be expressed in a variety of units. Parts per Million and Parts per Billion. P p m A M a s s o f A T o t a l m a s s o f t h e s o l u t i o n 10 6.
Martha has a large amount of 125 M H2SO4 in her lab. The gramme equivalent weight of a molecule is a measure of its reactive capacity. Free online concentration - solution converter - converts between 11 units of concentration - solution including kilogramliter kgL gramliter gL milligramliter mgL partmillion ppm etc.
This time though the final concentration is known you are asked to calculate the starting concentration. In the health sciences the concentration of a solution is often expressed as parts per thousand ppt indicated as a proportion. How many liters of the solution should she use.
If you solve for C you should get C A-bεm. Mass Concentration kgm 3 or gL - mass of solutevolume of solution. The concentration of a solution is a macroscopic property represents the amount of solute dissolved in a unit amount of solvent or of solution and can be expressed in a variety of ways qualitatively and quantitatively.
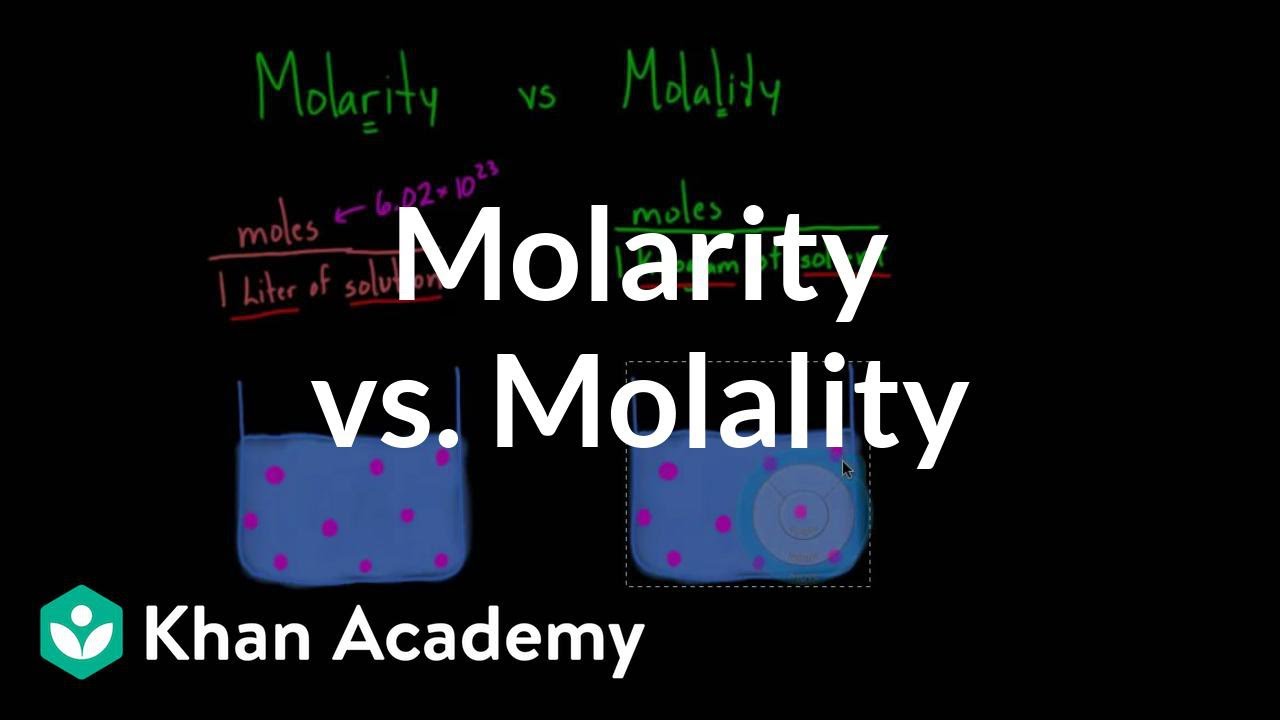
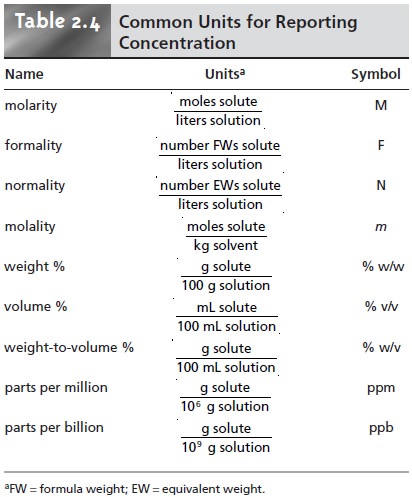
The standard unit is molar M which is the number of moles per unit volume molL. 1 molL 1 moldm 3 1 mol dm 3 1 M 1000 molm 3. Molality m - moles of solutemass of solvent not mass of solution Mass Percent - mass solutemass solution x 100 mass units are the same unit for both solute and solution.
This means for the above solution there should be 855 moles per 1 m 3 of the solution. This variation is a different way of asking about the same experimental dilution step that was originally described. Qualitatively solutions of relatively low concentration are described using adjectives such as dilute or weak while solutions of relatively high concentration are described as concentrated or strong As a rule the more concentrated a chromatic solution is the more intensely coloured it is.
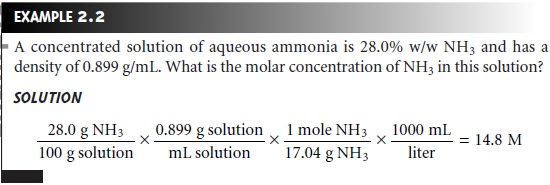
However molL is a more common unit for molarity. Give the concentration of the stock solution in units of grams per liter gL. Molar concentration gives the number of moles of the substance per unit volume in the mixture.
A mole of NaCl has a mass of 585 g. A solution that contains 1 mole of solute per 1 liter of solution 1 molL is called one Molar or 1 M. The SI unit for molar concentration is molm 3.
M εm slope or the molar extinction coefficient in beers law which has units of M-1cm-1 So A εmC b. As in the case of percentage concentration in parts per million can also be expressed as mass to mass volume to volume and mass to volume. The standard unit of concentration in chemistry is molarity abbrieviated with M.
Very low solute concentrations are often expressed using appropriately small units such as parts per million ppm or parts per billion ppb. Meant to be used in both the teaching and research laboratory this calculator see below can be utilized to perform a number of different calculations for preparing solutions having units per volume ie units over volume concentration units such as UnitsmL μUnitsμL mUnitsmL UnitsL etc. The four main categories of concentration units are mass concentration molar concentration number concentration and volume.
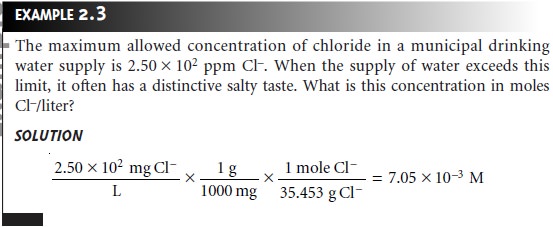
Concentration of solution fractextWeight of the solute in gramtextvolume in Litres We will also see other methods on how to calculate the concentration of a solution based on the different methods of expressing concentrations. X concentration C Note. Parts per billion ppb mass of solute mass of solution 109.
Most absorbance instruments come with a starter factory calibration for the parameters or compounds of choice which can be further refined on-site to improve the accuracy and reliability if necessary. Like percentage part per hundred units ppm and ppb may be defined in terms of masses volumes or mixed mass-volume units. There are a variety of different concentration units some of which express the same information in different ways and some of which provide different information about the relative amount of chemical that is within a mixture.
Concentration in Parts per Million. Show your work and enter your answer in the blank below to get full credit 008 moi 005L loemolil Molarity. What is the molality of this solution.
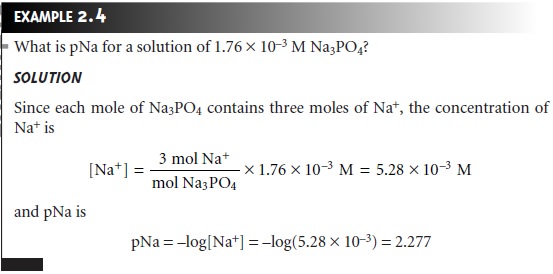
Qualitative Expressions of Concentration. The parts of a component per million parts 10 6 of the solution. The concentration of the solution formula is given as follows.
Molality is calculated using the formula. Depending on the nature of the discussion or study it can be useful to express concentration in other units. Also explore many other unit converters or learn more about concentration -.
Unit is M or molL. Normality N - grams active soluteliters of solution. She needs 36 grams of H2SO4 for a chemical reaction she wants to perform.
The unit molL can be converted to molm 3 using the following equation. When the concentration is expressed as the percent of one component in the solution by mass it. Parts per million ppm mass of solute mass of solution 106.
In the previous section we introduced molarity a very useful measurement. There are many different units of concentration. Mass percentage mass of solute mass of solution 100.
Therefore the concentration can be described as 855 mol m-3. For an example lets take the salt solution described above. A concentration measurement based on the gramme equivalent weight of the solute per litre of solution is known as normality.
Such concentration calculations are needed when starting with the. Lo v molL m 8. Define the concentration units of mass percentage volume percentage mass-volume percentage parts-per-million ppm and parts-per-billion ppb Perform computations relating a solutions concentration and its components volumes andor masses using these units.
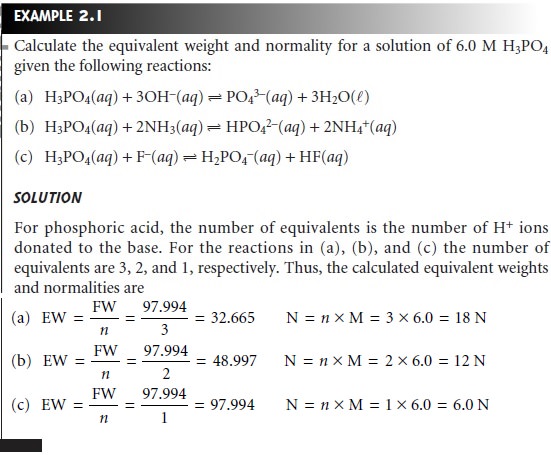
Molarity is calculated using the formula. EqL is the normality unit. What is a normality unit.
Moles Solute M Liter of Solution Calculate the molarity of the NaHCO solution in units of molL. Because of this relationship absorbance instrumentation can be programmed to output a concentration value in mgL or ppm using a calibration. Molarity is defined as moles of solute per liters of solution.
A solution can be qualitatively described as. A litre of seawater which weighs 1030 g contains about 6 10 3 g of dissolved oxygen O 2. A solution is prepared from 8 grams of acetic acid CH2CO2H and 725 grams of water.
Moles Solute Kg Solvent units of molkg. The letter N is used to represent normality.

Units Of Concentration Dilute Concentrated Solution Embibe

Plant Kingdom Powerpoint And Fill In Notes Learning Science Science Lessons Next Generation Science Standards
Concentration Units Chemistry Master
Solutions And Concentration Units Of Solutions Molarity Mole Fraction Molarity Formula And More

Units Of Concentration Dilute Concentrated Solution Embibe
Units For Expressing Concentration

Solutions And Concentration Units Of Solutions Molarity Mole Fraction Molarity Formula And More
Units For Expressing Concentration
Units For Expressing Concentration

Units Of Concentration Dilute Concentrated Solution Embibe

Kindergarten Weather Activities Weather Theme Weather Activities Weather Lessons

Convert Between Different Units Of Measurement In Windows Using This Portable Tool I Have A Pc Converter Portable Tools The Unit
Units For Expressing Concentration

How To Calculate The Concentration Of A Solution Chemistry Lessons Chemistry Education Teaching Chemistry

Significant Figures Videolessons Scientific Notation Chemistry Worksheets Teaching Chemistry

Concentration Math Games Bundle Math Centers Activity Math Games Math Center Activities Math Centers
Units For Expressing Concentration
Solutions And Concentration Units Of Solutions Molarity Mole Fraction Molarity Formula And More

Comments
Post a Comment